
In a second experiment it took 32.93 mL of 0.100 M HCI to titrate completely the NH3 present in 0.341 g compound A. When 0.105 g of compound A was strongly heated in excess 0 2, 0.0203 g CrO3 was formed. Since it carries a #color(blue)(3+)# charge, it follows that you must use the (III) Roman numeral. A solid is isolated (compound A), and the following data are collected: i. Cr2(CO3)3 wales timber mold chromium iii hyrogen. #color(red)(2) xx "Cr"^(color(blue)(3+)" "# and #" " color(blue)(3) xx "CO"_3^(color(red)(2-)#īecause chromium is a transition metal, which means that it can have multiple oxidation states, you must use Roman numerals to express its oxidation state in the compound. Cations are always written first in the chemical formula of an ionic compound, followed by the anions. This means that the ions that make up this compound are Round your answer to the nearest percentage. The only compound with the molecular formula CX3CrOX9 C X 3 C r O X 9 to be found is tricarbonatochromate (III). STOICHIOMETRY Finding mass percent from chemical formulae This is the chemical formula for chromium (III) carbonate: Cr2 (CO3), Calculate the mass percent of carbon in chromium (III) carbonate. orthocresol at 23:10 2 Scifinder agrees with ortho. This means that the charge on the cation will be equal to #color(blue)(3+)#. Fairly sure this thing doesn't exist to begin with - it would immediately decompose to CrOX3 +3COX2 C r O X 3 + 3 C O X 2. Notice that it's written between parentheses, which tells you that it contains one atom of carbon and three atoms of oxygen, and that it has a subscript of #color(blue)(3)#.
#Chemical formula for chromium iii carbonate full
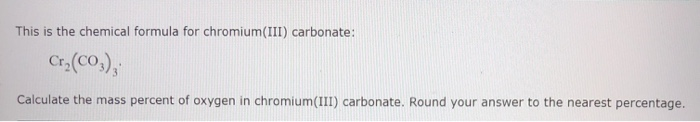
Now, the anion is actually a polyatomic ion called the carbonate anion. Provied information about Chromium(III)carbonate,basic(Molecular Formula: CCrO3+, CAS Registry Number:5 ) ,Boiling Point,Melting Point,Flash Point. Expert Answer 100 (3 ratings) Answer: Given : 1) Chemical Formula Cr2 (CO3)3 2) mass percentage of oxygen in Cr2 (CO3)3 We know that, Mass percenta View the full answer Transcribed image text: This is the chemical formula for chromium (III) carbonate: Cr2 (CO3), Calculate the mass percent of oxygen in chromium (III) carbonate.
Since it has a subscript of #color(red)(2)#, it follows that the charge of the anion must be equal to #color(red)(2-)#.

In your case, the compound contains chromium, #"Cr"#, as its cation. It's important to remember that ionic formulas are written using the crisscross rule, which states that when a cation and an anion form an ionic compound, the charge on the cation becomes the subscript of the anion and the charge of the anion becomes the subscript of the cation in the chemical formula of the compound. In order to name an ionic compound, you must identify the cation, which is the positively charged ion, and the anion, which is the negatively charged ion.Ĭations are always written first in the chemical formula of an ionic compound, followed by the anions.


 0 kommentar(er)
0 kommentar(er)
